
Navigating FDA Labeling Requirements for CBD Products
Our friends at Ananda Hemp shared this excellent article to provide some insight into labeling requirements for hemp and CBD products!
The CBD market is considered to be a growing industry. The growth potential in the United States alone could reach billions in sales. This obviously means a growth in the use of cannabidiol products as well. This being said, businesses should be particularly considerate of the packaging and labeling of CBD products. It can be challenging to navigate labeling CBD and hemp CBD products since there are no CBD-specific regulations prescribed by the federal government. It is important to be updated with requirements and local regulations when it comes to packaging and labeling these CBD products since these regulations can quickly change nationwide.
GETTING TO KNOW CANNABIDIOL (CBD)
In the United States, cannabidiol or CBD is marketed in foods, dietary supplements, cosmetics, and several tobacco products, all of which are mainly regulated by the Food and Drug Administration or the FDA. The FDA is responsible for the safety and efficacy of foods, drugs, cosmetics, biological products, and medical devices. The FDA continues to believe that the drug approval process is one of the most ideal ways to ensure the safety and effectiveness of products.
CBD is the second most common ingredient of cannabis. It is derived from the hemp plant, which is considered to be the cousin of the marijuana plant since they are the same species. Although CBD is a component of marijuana it does not produce the “high” that is associated with it. It is said that there are a wide range of health benefits related to CBD. However, the one that has the most potent evidence would be its effectiveness in treating two rare and severe forms of epilepsy. The only FDA approved medical product that contains cannabis is Epidiolex. Cannabidiol is the active ingredient in Epidiolex, the only pharmaceutical drug containing CBD that has been approved by the FDA. It is a prescription drug used to help treat seizures related to the Lennox-Gastaut Syndrome and Dravet Syndrome, two rare and severe forms of epilepsy.
WHAT ARE THE DIFFERENCES BETWEEN HEMP CBD AND CANNABIS CBD?
One of the frequently asked questions about this topic would be about the differences between hemp derived cannabidiol and cannabis CBD. In order to gain a better understanding, this article will also help clarify information about these two. The 2018 Farm Bill defines hemp as the plant Cannabis Sativa L. that contains less than 0.3% THC or the CBD cannabinoid component. THC is known to be the component that produces the “high” as a result of marijuana use. The enactment of this bill also removes hemp, from the Controlled Substances Act under federal law. On the other hand, Cannabis plants have three varieties which are Cannabis Indica, Cannabis Sativa, and Cannabis Ruderalis. These plants contain significantly higher levels of THC as compared to hemp plants and contain less cannabidiol. Basically, hemp CBD contains more CBD and less THC, while cannabis CBD contains more THC and less CBD. Other products including CBD include oils, tablets, softgels, topical salve, lotion, massage oil, etc. The marketing and sale of these products are legal in the United States but with different restrictions per state and from the FDA.
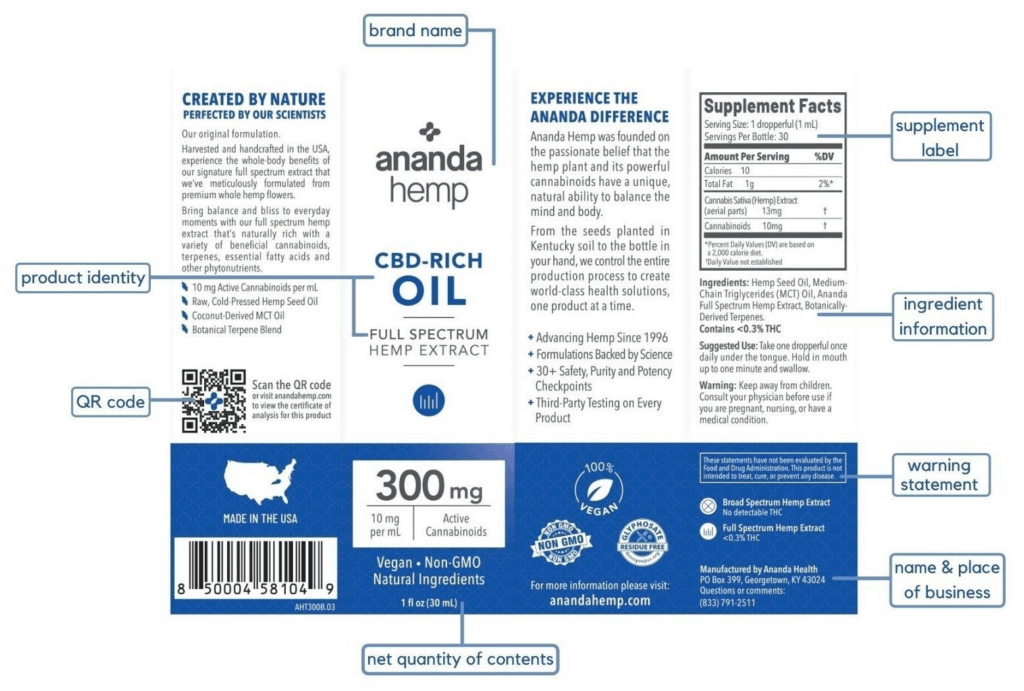
GENERAL LABELING REQUIREMENTS OF THE FDA FOR CBD PRODUCT
CBD Companies should consider the best packaging choices for their products. These kinds of packaging include child-resistant containers, glass bottles, and airtight packaging in colored containers, to ensure that contents are protected from artificial light or UV rays.
Moving on to labeling, the FDA has specific regulations for the use of supplement labels and food labels. This also covers regulations that include food products including those related to food additives. Products that contain CBD are subject to different labeling regulations. States that allow the sale of CBD products, although having varying regulations, require companies to strictly follow CBD labeling regulations that are in accordance with the Federal Food, Drug, and Cosmetics Act, also known as the FDCA. The FDCA requires several basic elements when labeling CBD products, which we will be getting to know more about in this section of the article.
- Identity Statement – Also known as product identity, this refers to what the product is or what it does. It should be placed in the front or in the product display panel where it can be easily seen. It is important to specify whether they are dietary supplements, a food additive, etc. Above is an example of a product label from Ananda Hemp. It can be clearly seen on the packaging that the product identity is “CBD-rich oil, full spectrum hemp extract.”
- Net Quantity of Contents – It is considered a basic requirement to state the exact amount of the product without its container. It is essential as well to indicate the exact amount of a specific nutrient (e.g. hemp extract, CBD oil, etc.).
- Responsibility Statement – This refers to the name and place of the business. This vital element will give the consumers a way to contact the manufacturer or distributor if necessary. It would be useful to include a contact number and website they can visit for more information.
- Ingredient Information – This is an ingredients list that includes all of the ingredients your product contains.
- Supplement Label – Also known as “Supplement facts” or “Nutrition facts”. Nutrition labeling and supplement labels should include key elements like serving size and amounts of things like sugars and fats, if applicable, as well as specific details about the active supplement ingredients.
- Warning Statement – It is necessary to include warning or caution statements that indicate that these products have not been subject to FDA evaluation. Make sure that there are no medical or health claims (e.g. this product can treat, cure, or prevent serious diseases, etc.). The FDA makes it a point to issue warning letters that market CBD products to firms that make false therapeutic or medical claims.
- QR Code – This QR code will direct consumers to a document that includes information such as the product name, expiration date, batch identification number, batch date, batch size, total quantity produced, ingredients used, and the certificate of analysis.
- Suggested use – Provide instructions with the appropriate dosage and application or consumption method.
Companies that market CBD products have much to remember when labeling Cannabidiol CBD products and Hemp CBD products aside from making sure that the packaging of the products is compliant with the guidelines set by the FDA as well. The previously mentioned elements are the essential information included when packaging and labeling your products. Make sure to always review packaging guidelines and that all the information indicated on the label is updated and accurate.
THE LATEST FROM INOVAR
WE'RE HERE TO SERVE YOU
Creating and producing labels can be overwhelming, but our experts are here to guide you every step of the way. Whether you have a project ready to go, have questions about label applications or materials, or want to learn more about our services, our team is ready to assist you.


